
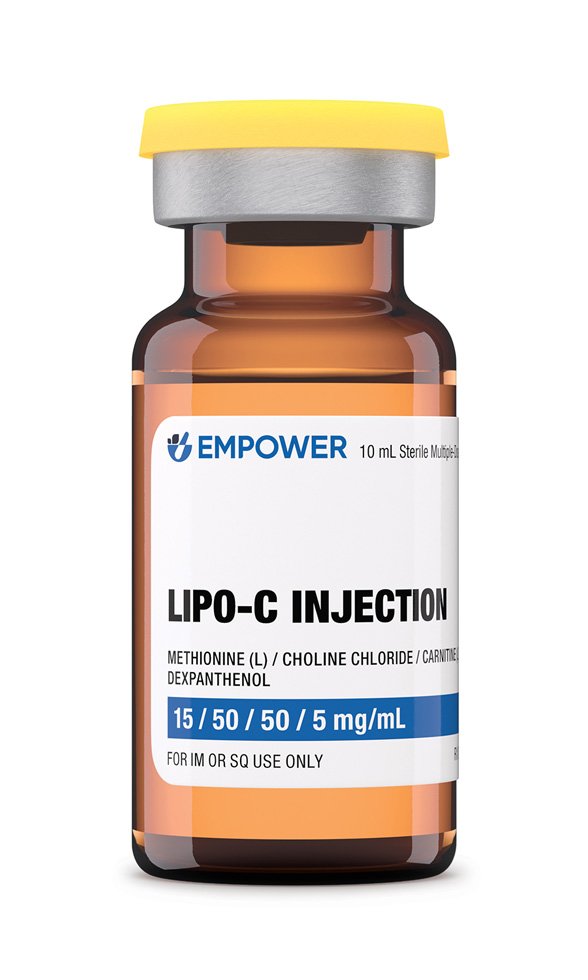
Lipo-C Injection is a sterile intramuscular formulation that combines four lipotropic and metabolic cofactors-methionine, choline chloride, L-carnitine, and dexpanthenol-at 15 mg/mL, 50 mg/mL, 50 mg/mL, and 5 mg/mL, respectively, in water for injection. It is offered in 30 mL and 10 mL multidose vials, providing clinicians and patients with flexible inventory for individualized treatment programs that aim to potentially support hepatic fat mobilization, potentially enhance mitochondrial fatty-acid oxidation, and potentially improve overall energy metabolism in adults pursuing physician-supervised weight-management or wellness regimens.
Because the ingredients are compounded rather than supplied as an FDA-approved fixed-dose product. The formulation’s uniqueness lies in delivering synergistic quantities of methyl-donor amino acids (methionine and choline), a carnitine shuttle cofactor, and a provitamin B₅ derivative in one injection, thereby bypassing gastrointestinal absorption limits that may hinder oral supplementation. Clinicians often integrate the product with calorie-restricted diets, resistance training, and behavior-change counseling to potentially maximize adipose mass reduction while preserving lean tissue, though evidence remains largely extrapolated from the individual components’ pharmacology and not from randomized controlled trials of the exact compounded combination.
A common starting regimen in adults with normal renal and hepatic function is 1 mL intramuscularly once or twice weekly, titrated every four weeks according to biochemical markers such as fasting lipid panel, liver transaminases, and serum carnitine. Dosage may be reduced to 0.5 mL weekly in patients experiencing gastrointestinal intolerance or increased to a maximum of 2 mL twice weekly for short-term metabolic intensification when supervised by a qualified clinician.
If a scheduled injection is missed, the patient should administer the dose as soon as remembered unless it is within 24 hours of the next scheduled dose, in which case the missed dose is omitted to prevent overlap. Therapy is ordinarily continued for 12 to 16 weeks before reassessment; maintenance beyond this interval should be individualized based on anthropometric response and laboratory safety indicators.
Methionine donates methyl groups for the synthesis of S-adenosyl-methionine, which in turn drives phosphatidylcholine production and very-low-density lipoprotein assembly, facilitating export of triglycerides from hepatocytes and mitigating diet-induced steatosis.
Choline chloride serves as an essential substrate for phosphatidylcholine and betaine formation, thereby enhancing bile-dependent lipid emulsification and acting as an osmolyte that maintains hepatocyte membrane integrity under oxidative stress; adequate choline intake has been shown to lower alanine aminotransferase levels and improve hepatic insulin sensitivity in clinical studies.
L-carnitine transports long-chain fatty acids across the inner mitochondrial membrane as acyl-carnitine esters, permitting β-oxidation and ATP generation while simultaneously buffering toxic acyl-CoA accumulation; supplementation has demonstrated increased maximal oxygen consumption and modest weight loss in meta-analyses of overweight adults.
Dexpanthenol, the stable alcohol analogue of pantothenic acid, is rapidly converted to coenzyme A, a co-substrate for acyl-group transfer reactions in both fatty-acid and carbohydrate metabolism. By bolstering coenzyme A pools, dexpanthenol may heighten Krebs-cycle flux and attenuate exercise-induced fatigue. When co-administered with methyl donors and carnitine, it provides the acetyl acceptor capacity necessary for sustained mitochondrial oxidation, creating a complementary, multi-pathway approach to lipid mobilization and energy production.
Use of Lipo-C Injection is contraindicated in patients with known hypersensitivity to methionine, choline salts, carnitine, dexpanthenol, or any formulation excipients, as well as in individuals with severe hepatic failure in whom augmented methionine intake could precipitate hypermethioninemia and encephalopathy. Pre-existing inborn errors of sulfur-amino-acid metabolism (e.g., homocystinuria) represent an absolute contraindication because parenteral methionine can exacerbate elevated homocysteine concentrations and associated thrombotic risk.
Caution is advised in patients with advanced renal impairment or those receiving dialysis, given the renal clearance of carnitine and the risk of accumulation; dose spacing or serum-level monitoring may be necessary. Clinicians should also avoid or adjust therapy in individuals with active peptic ulcer disease due to theoretical choline-mediated increases in gastric volume and in those receiving levodopa, as pantothenic-acid derivatives may interfere with levodopa absorption. Patients with severe heart failure or arrhythmias require monitoring because rapid shifts in free-fatty-acid metabolism can influence myocardial substrate utilization.
High-dose methionine may antagonize the therapeutic effect of L-dopa-carbidopa regimens by increasing peripheral methylation of the drug complex, potentially reducing central bioavailability; therefore, neurologists often recommend spacing Lipo-C Injection and antiparkinsonian doses by several hours. Concurrent administration with cysteine or N-acetylcysteine enhances hepatic sulfate production, which could alter acetaminophen metabolism and should be considered when treating patients with chronic pain requiring frequent analgesia.
L-carnitine has been reported to potentiate the anticoagulant effect of warfarin through unknown mechanisms, with case reports documenting supratherapeutic international normalized ratios after initiation of parenteral carnitine in malnourished adults. Choline may increase cholinergic tone and could theoretically augment adverse reactions when combined with acetylcholinesterase inhibitors such as donepezil; clinicians should monitor for gastrointestinal cramping or bradycardia in co-treated patients.
The most frequently observed adverse reactions are mild injection-site pain, transient erythema, and localized warmth, typically resolving within minutes; rates of these events are comparable to other aqueous vitamin injections and rarely necessitate treatment interruption.
Gastrointestinal disturbances-including nausea, loose stools, and a transient “fishy” body-odor attributable to trimethylamine production from excessive carnitine and choline metabolism-occur in a minority of recipients and are usually self-limiting within 24 to 48 hours after injection.
Serious but uncommon events include immediate-type hypersensitivity with urticaria, bronchospasm, or anaphylaxis, and delayed eosinophilic myositis. In a retrospective series of over 2,000 compounded lipotropic injections, the incidence of systemic hypersensitivity was below 0.1 percent, yet clinicians should maintain readiness to administer intramuscular epinephrine and transfer patients to higher-level care when indicated.
Parenteral choline supports fetal neurodevelopment, yet the safety of supraphysiologic methyl-donor combinations in pregnancy lacks robust evidence; professional guidelines thus recommend limiting Lipo-C Injection to cases in which potential maternal benefit outweighs theoretical teratogenic risk, and only under obstetric supervision.
Methionine and carnitine are present in breast milk in concentrations reflective of maternal plasma levels; while neither compound is known to impair lactation, the concentrated doses delivered by weekly injections may alter milk taste or infant gastrointestinal tolerance. Lactating individuals considering Lipo-C Injection should discuss risk-benefit considerations with a pediatrician and undergo periodic infant growth monitoring.
Lipo-C Injection should be stored at controlled room temperature-20 to 25 °C (68 to 77 °F)-and protected from light in its original carton. Do not freeze or expose to excessive heat. Visual inspection for particulate matter or discoloration is required before administration; compromised vials should be discarded according to local disposal regulations.
Do not freeze the product and avoid vigorous shaking to minimize foaming that can entrap air and compromise dose accuracy. The beyond-use date is 28 days from the compounding date when prepared under USP <797> medium-risk conditions; any residual solution remaining after this interval, or if contamination is suspected, must be discarded in accordance with local pharmaceutical-waste regulations.